



| Place of Origin: | Guangdong, China |
| Brand Name: | OBS |
| Model Number: | #0040 |
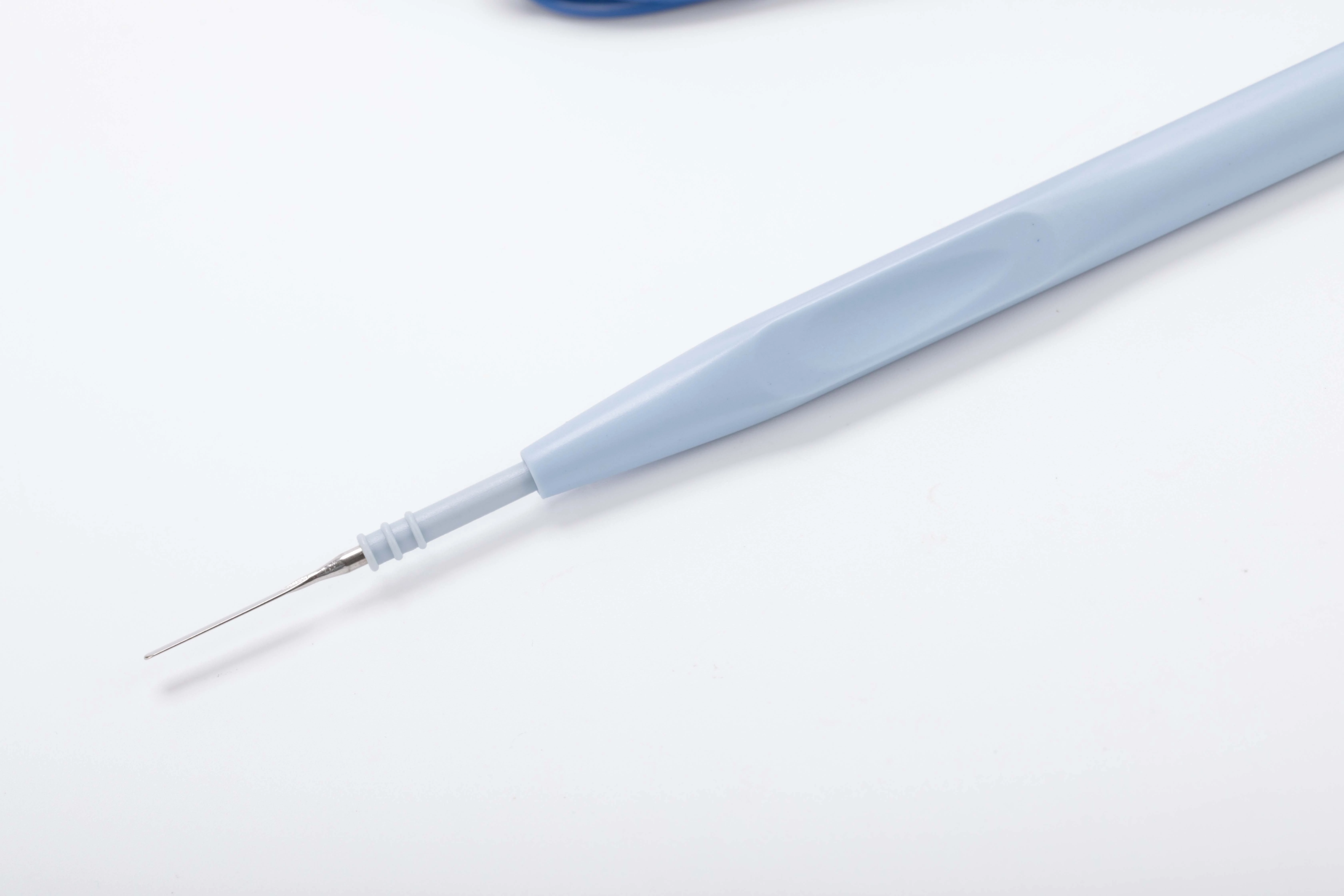
| Properties: | The Basis of Surgical Instruments |
| Type: | Knife |
| Mode: | OBS-Df #0048 |
| Color: | Grey |
| Sterilization: | EO |
| Shell Lenght: | 15.3cm |
| Cable Lenght: | 3m/5m |
| Plug: | 5.3 plug/6.3(HIFI) Plug |
| Weight Per Piece: | 0.068kg |
Video Description
Match with Electrosurgical Generator(Unit), Radio Frequency Generator(Unit) and other High Frequency Equipment.
D: Disposable
These devices are designed to be used as accessories in conjunction with those electrosurgical units and electrodes. These devices are single use supplied sterile. Check plug matching on generator base and connect. Maximum voltage is not be exceed 90KV peak
Foot control design relieves the surgeon's hands in the surgery, and prevents accidental activation of the device.Designed with the surgeon in mind
-Slim and lightweight, tapered ergonomic design, streamlined design,
-Slip-proof handling
-With hexagonal shape to protect against inadvertent twisting, the electrodes can be locked safely into place and are easily exchanged and replaced.


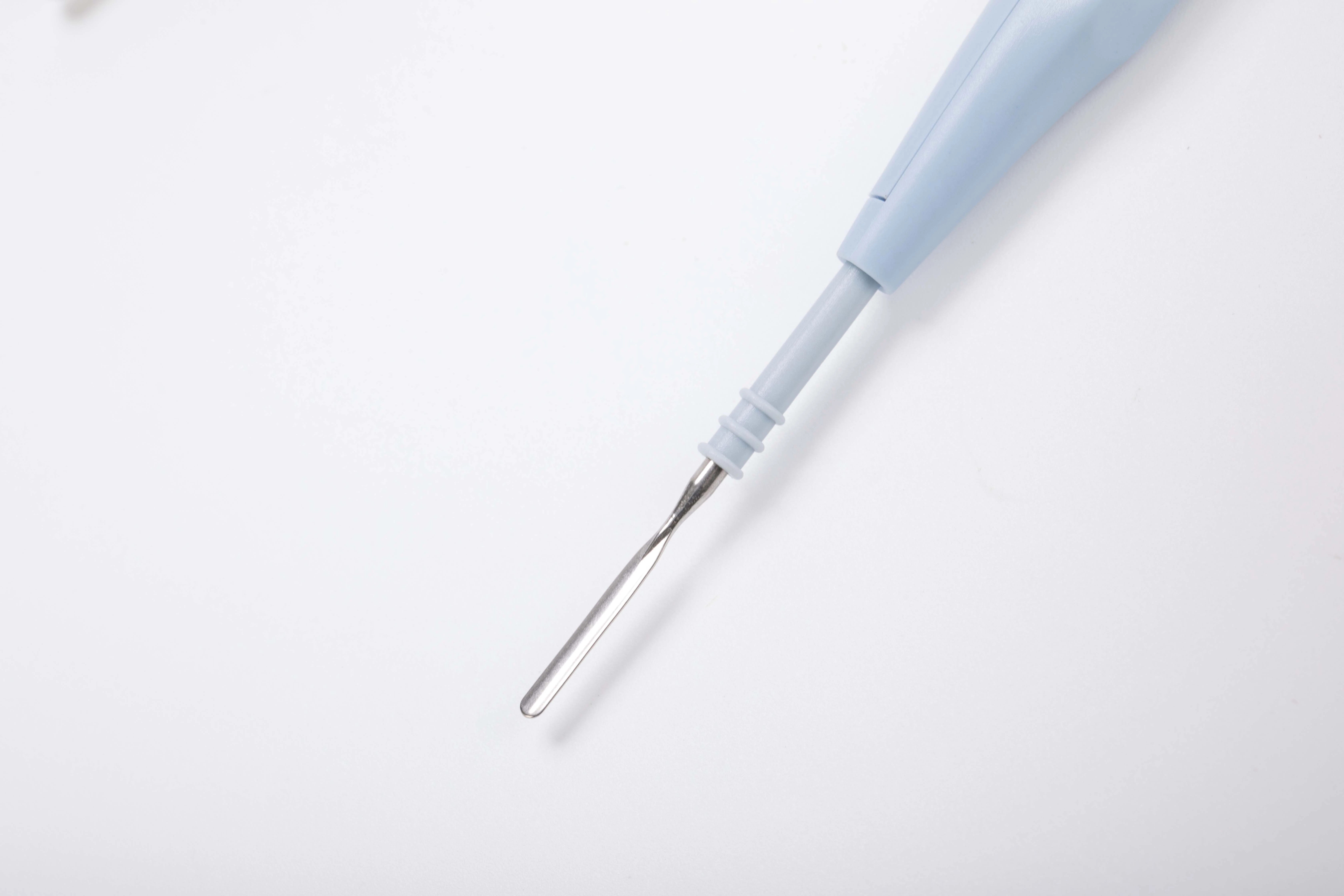
Electrical Performance Data
Maximal Output Current | Frenquency Confine | Current Resistence | Output Power |
1A | 0-1.0MHz | 200Ω | 50-80W |
These devices should never be used when:
There is visible evidence of damage to the exterior of the device such as cracked or damaged plastic or connector damage.
These devices fail the inspection described herein.
In the presence of flammble gases, flammable prep solutions or drapes. Oxidizing. Gases such as Nitrous Oxide (N2O),or in oxygen-riched environments.
Do not used monopolar electrosurgery on small appendages, as in circumcision or finger surgery.
Content | Net Weight | Gross Weight | Carton Size | |
Paper Packaging | 100 pcs/ctn | 6.8kg | 7.3kg | 36*27*28 |
Blister Packaging | 25 pcs/ inner ctn; 4 inner ctns/outer ctn | 9.8kg | 11.5kg | 53.5*36*40 |